Technology
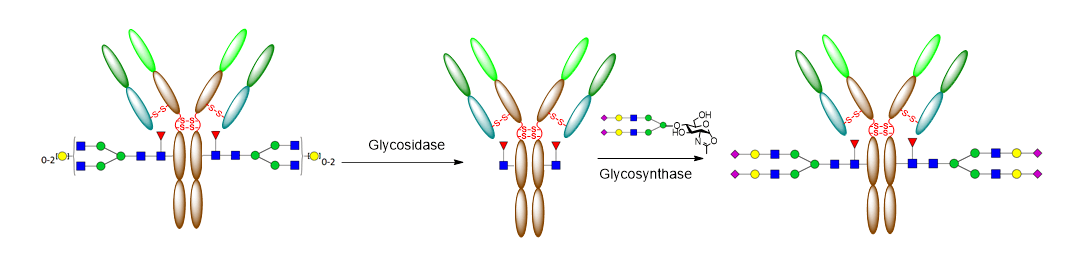
The key technology of GlycoT (developed by Prof. Wang’s group in University of Maryland) permits chemoenzymatic glycoengineering of therapeutic antibodies and other glycoprotein products to provide structurally well-defined, homogeneous glycoforms with unique properties. This technology development has resulted in over a dozen of US patent applications, including 6 issued US patents and 4 international patents. As demonstrated for the glycosylation remodeling of antibodies, this technology consists of two key steps: deglycosylation of the antibody by an endoglycosidase to remove the heterogeneous Fc glycans in a single step and subsequent attachment of a desired N-glycan to the deglycosylated antibody by a novel glycosynthase to reconstitute the antibody with a defined glycan. This technology opens a new avenue to accessing a wide range of homogeneous antibody glycoforms that would be otherwise difficult to obtain by other chemical or biological approaches. The IPs cover the novel enzymes/mutants, the process, and the products.
Selected Reference
- Li C, Zhu S, Ma C, and Wang LX, “Designer a1,6-Fucosidase Mutants Enable Direct Core Fucosylation of Intact N-Glycopeptides and N-Glycoproteins”, J. Am. Chem. Soc., 139, 15074-15087 (2017).
- Yang Q, An Y, Zhu S, Zhang R, Loke CM, Cipollo JF, Wang LX, “Glycan Remodeling of Human Erythropoietin (EPO) Through Combined Mammalian Cell Engineering and Chemoenzymatic Transglycosylation”, ACS Chem. Biol., 12, 1665-1673 (2017).
- Li, T., DiLillo, D., Bournazos, S., Giddens, J. P., Ravetch, J. V., Wang, L. X., “Modulating IgG effector functions by Fc glycan engineering”, Proc. Natl. Acad. Sci. USA, 114, 3485-3490 (2017).
- Yamaguchi, T., Amin, M.N., Toonstra, C., Wang, L.X., “Chemoenzymatic synthesis and receptor binding of mannose-6-phosphate (M6P)-containing glycoprotein ligands reveal unusual structural requirements for M6P receptor recognition”, J. Am. Chem. Soc., 138, 12472-85 (2016).
- Li, T., Tong, X., Yang, Q., Giddens, J.P., Wang, L.X., “Glycosynthase mutants of endoglycosidase S2 show potent transglycosylation activity and remarkably relaxed substrate specificity for antibody glycosylation remodeling”, J . Biol. Chem., 291, 16508-16518 (2016)
- Giddens, J.P., Lomino, J.V., Amin, M.N., Wang, L.X., “Endo-F3 glycosynthase mutants enable chemoenzymatic synthesis of core fucosylated tri-antennary complex-type glycopeptides and glycoproteins”, J. Biol. Chem., 291, 11064-11071 (2016).
- Amin, M.N., McLellan, J.S., Huang, W., Orwenyo, J., Burton, D.R., Koff, W.C., Kwong, P.D., Wang, L.X., “Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies”, Nature Chem. Biol., 9, 521-526 (2013).
- Huang, W., Giddens, J., Fan, S.Q., Toonstra, C., Wang, L.X., “Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions”, J. Am. Chem. Soc., 134, 12308−12318 (2012).